Announcer:
Welcome to CME on ReachMD. This activity is brought to you by Med-IQ and supported by an educational grant from Daiichi Sankyo, Inc. Before starting this activity, please be sure to review the faculty and commercial support disclosure statements as well as the learning objectives. Here’s your host, Dr. Jennifer Caudle.
Dr. Caudle:
HER3 has been implicated as a potential therapeutic target due to its high expression levels and many tumor types and presence of genetic mutations in several tumors. This podcast will explore HER3-targeted treatments and break down their mechanism of action, efficacy, and treatment. This is CME on ReachMD, and I’m your host, Dr. Jennifer Caudle. I’d like to welcome Dr. Sara Hurvitz and Dr. Narjust Florez to the program, who are joining me to discuss targeting HER3 in breast and lung cancers. Dr. Sara A. Hurvitz is Professor of Medicine and Director of the Breast Cancer Clinical Trials Program at UCLA’s David Geffen School of Medicine in Los Angeles, California. Dr. Narjust Florez is an Assistant Professor of Medicine at Harvard Medical School in Boston, Massachusetts. She’s also Associate Director of the Cancer Care Equity Program at Dana-Farber Cancer Institute. Dr. Hurvitz and Dr. Florez, welcome to you both.
Dr. Hurvitz:
Thank you.
Dr. Florez:
Thank you.
Dr. Caudle:
Let’s start with a brief background on HER3. Dr. Florez, can you explain more of the rationale for targeting HER3 in cancers?
Dr. Florez:
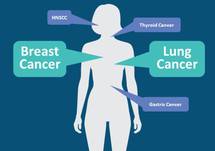
Yes. So, before we start talking about HER3 independently, let’s talk about the family that HER3 belongs to. So, HER3 belongs to the human epidermal receptor family. Receptor tyrosine kinases including EGFR, HER2, HER3, and HER4. So, they transduce growth-promoting signaling in response to ligand binding to their extracellular domains. Why is it important to understand where HER3 comes for? Because a lot of the mechanisms of resistance are to the other family of the same family. This family of human epidermal receptors are deregulated in numerous cancers, as we mentioned, and HER3 particularly is highly seen in breast cancer as well as non-small cell lung cancer where it also has been described in other malignancies, including gastric cancer and thyroid cancer as well. So, while we are talking about HER3, we need to acknowledge that we have paid less attention to this pathway. However, recent work has placed the spotlight in HER3 because of several clinical contexts. First, HER3 has been shown to play a major role in mediating resistance to HER2 and PI3-kinase pathway directed therapies due to its feedback regulation via the Akt signaling. Second, activating mutations in HER3 have recently been identified in multiple cancer types, as we mentioned, and also have been described as a resistance after you have used the adequate target. Finally, HER3 is directly associated with tumorigenesis. The activation through ligands and dimerization induces metastases as well. Cell migration, and in many cancers, HER3 activation have been seen as a negative predicted factor. It’s important to note that HER3 is one of the emerging biomarkers as a first line or at the time of diagnosis and also as resistant mechanisms, and we are going to continue to hear about HER3 for more years to come.
Dr. Caudle:
So, next let’s walk through the consequences of expression, overexpression, or mutation of HER3 specific to breast and lung cancers. Dr. Florez, please talk with me about the consequences of HER3 mutations in lung cancer.
Dr. Florez:
All right. So, let’s talk about this because I think it’s very important and we have to backtrack to EGFR again as the beginning. So, what we know most about HER3 and lung cancer comes with the resistant mechanisms associated with resistance to EGFR-directed therapy. So, the HER3 pathway is an escape pathway that has been seen in patients that have a permanent irreversible inhibition of the EGFR pathway from the external aspect – it means through drugs. So, we do know that these patients, we have their resistant mechanisms associated, and that’s why there’s a lot of lines of research there. So, first thing about lung cancer in HER3 – resistant mechanism. Second aspect for HER3 in lung cancer is the HER3 messenger RNA expression. It’s associated – we have decreased survival rates. And why is that important? We do know that HER3 tends to induce metastases of cell migration in tumorigenesis. So, this mutation has two negative aspects in lung cancer. Since the get go from diagnosis, it tends to be a more aggressive disease, the patients have to have less survival and worse outcomes, and also we see HER3 as a mechanism of resistance, so expression at two different phases of lung cancer, and it’s associated with the same family, right? It’s the HER family. EGFR is part of the family, HER2 lung cancer is part of the family, so HER3 also serves as a mechanism of resistance there, and as a whole, when there’s RNA expression, there is decreased overall survival for these patients.
Dr. Caudle:
And Dr. Hurvitz, what about within breast cancer?
Dr. Hurvitz:
Within breast cancer, HER3 overexpression is actually quite common not only in HER2-positive breast cancer where it’s been described widely, but also in hormone receptor–positive and triple-negative breast cancer. Transgenic mammary tumor models have demonstrated that inhibition of HER3 revokes HER2-dependent tumorigenesis. We know in breast cancer that HER3 expression has been linked to increased risk of metastasis. 30% of primary tumors have been shown in metastatic breast cancer to express HER3, and 60% in matched metastatic samples, suggesting that expression is linked to metastatic events. We know that HER3 expression has been associated with worse overall survival and a higher risk of death than in patients whose tumors fail to or do not express HER3, and HER3 signaling has also been implicated in the resistance to hormone therapy, HER2-targeted treatment, chemotherapy, and radiation therapy in breast cancer specifically. So, all of this, in summary, indicates that HER3 may be playing a role in the behavior of breast cancers when expressed. As Dr. Florez mentioned earlier, HER3 is implicated in transactivation of the PI3-kinase pathway, and this has been shown to be a mechanism of resistance to HER2-targeted therapies as well as endocrine-based therapies, both of which are very important in the treatment of breast cancers. Given all of these findings – the association with prognosis and implications in disease progression and resistance to treatment – it’s become a natural target that investigators have looked at to see whether inhibiting it or blocking it or using it to gain access to tumor cells would be beneficial in breast cancer in particular.
Dr. Caudle:
This is clearly an important therapeutic target. How are novel agents targeting HER3 in breast and lung cancer? Generally, what are their mechanisms of action and how do they work?
Dr. Hurvitz:
A number of therapies are in development that target HER3 in breast cancer and lung cancer as well as other solid tumors. In lung cancer in particular, activating mutations in HER3 have been identified, as well as in other tumors, and this may play a major role in mediating resistance to other directed therapies, including PI3-kinase pathway inhibitors and HER2 blockade. So, a number of drugs are being evaluated to block HER3 at its dimerization’s partner’s kinase activity, blocking its most relevant dimerization partner’s ability to dimerize with HER3, and directly targeting the HER3 extracellular domain. In addition, monoclonal antibodies have been the main drug modality to target HER3. This blocks the HER3 ligand and/or other HER receptor interactions. In addition to monoclonal antibodies, vaccines are being evaluated that target HER3 and incite immune responsiveness against HER3-expressing cancers, and antibody drug conjugates are in development. Antibody drug conjugates utilize HER3 to target a cytotoxic payload to the HER3-expressing cancer.
Dr. Caudle:
For those of you who are just joining us, this is CME on ReachMD. I’m your host, Dr. Jennifer Caudle, and joining me to talk about targeting HER3 in breast and lung cancers are Dr. Sara Hurvitz and Dr. Narjust Florez.
So, doctors, based on the clinical evidence you shared with us earlier, let’s walk through the efficacy and safety of some of the most promising medications targeting HER3 thus far. Let’s start with patritumab deruxtecan. Dr. Florez, can you tell me about the mechanism of action and studies?
Dr. Florez:
Well, to all our listeners, these are the two years of ADCs, right? These are the new kids on the block. People are very excited about ADCs. So, we’re seeing a lot of advancement for ADCs in lung cancer, and we’re going to talk a little bit about the efficacy and safety of HER3-targeting agents, particularly an antibody-drug conjugate. So, let’s talk about the efficacy of this medication, patritumab deruxtecan. This is a HER3-targeted ADC, so it’s composed of a drug linker containing deruxtecan, which is a topoisomerase 1 inhibitor, and the antibody, the patritumab. So, I want you to, our listeners, imagine an ADC is composed of three parts. First, we have the antibody that we attached to the HER3, we have the linker, and then we have the payload that in this case is a topoisomerase 1 inhibitor. So, when you try to imagine these agents, the antibody attaches to the antigen – the HER3 antigen – then the drug linker allows to internalize the chemotherapy to the cell level. I love it because when I explain this to my patients, it sounds like it came from the Jetsons, right – this type of medication. So this is done to, of course, inhibit the growth of HER3-positive cancers, and the majority anti-tumor activity is related to the cytotoxic payload, but for ADCs, it’s a little bit more complex. There are many factors that play a role. First, the antigen: This drug needs to find the antigen in the HER3-expressing cells, right, in the cell surface. Also, how is the ADC internalized into the cell? How is the cell welcoming this toxic medication? Of course, these – I want you to imagine the space mission leaves behind parts of the aircraft. So, the enzymatic cleavage allows the drug to come intracellular and deliver the payload. So, we have studied HER3, particularly patritumab deruxtecan, in patients that have EGFR-mutant non-small cell lung cancer that have developed resistance to EGFR inhibitors, particularly in these patients that have that escape mechanism at the time of disease progression, and that brings another important point, which is biopsy at the time of progression in lung cancer to identify the resistant mechanism. But in this clinical trial, that is studied, and it was recently presented, these 57 patients received the HER3 ADC 5.6 milligrams per kilogram every three weeks, so very standard dosing, and we had an objective response rate of around 39% with a median progression-free survival of 8.2 months. So, when you see these drugs are for HER3-positive non-small cell lung cancer, patients with EGFR non-small cell lung cancer that have resistance, and with an emerging biomarker, which is NRG1. This is extremely important because we have very limited options for patients that have disease progression after EGFR therapy, and EGFR non-small cell lung cancer is the second most common biomarker in lung cancer. So, let’s talk about some of the side effects. The side effects are associated – I want you to imagine what happens when a monoclonal antibody marries chemotherapy – those are the side effects that we see. We see bone marrow suppression with neutropenia, thrombocytopenia, we have fatigue, but something that’s very unique to antibody drug conjugates is interstitial lung disease. So, around 4 patients of these 57 patients, so around 9%, had associated drug-related interstitial lung disease. So, these patients need to be followed very closely. There were no drug-related deaths. So, overall, the side effects are bone marrow suppression, we also can see interstitial lung disease in these patients, fatigue. I put patients on these drugs – fatigue can be very, very common.
Dr. Caudle:
Great, thank you. Now, let’s talk about zenocutuzumab. Dr. Hurvitz, can you tell me more about the mechanism of action and latest results for this agent?
Dr. Hurvitz:
We are seeing some really exciting data relating to patritumab deruxtecan for breast cancer as well. There was actually a very nice study presented by Ian Krop and colleagues, which was an 182 pooled patient analysis. It was a phase 1/2 multicenter study, and they enrolled patients who had HER2-positive, HER3-positive breast cancer, and triple-negative breast cancer and hormone receptor positive, HER2-negative, HER3-high and -low breast cancer, so a huge cohort of patients, to evaluate the safety and efficacy in breast cancer in particular with this novel HER3-targeted ADC. What was interesting in this study was the objective response rates seen were quite notable in a very heavily pretreated patient population where patients who enrolled had had a median of 5 prior lines of therapy. The range of prior therapies actually went up to 13, so very heavily pretreated patient population, and the objective response rates seen in HER2-positive was about 43%. It was about 30% for hormone receptor–positive, HER2-negative breast cancer, and 23% for triple-negative breast cancer with high expression of HER3. I think one thing that we’re going to be looking at as we see new studies evaluating this novel ADC is the level of HER3 expression that’s needed to exert the efficacy of this agent. In this particular clinical trial, they deemed a tumor to be HER3-high if it had at least 75% membrane positivity for HER3 and HER3-low if it was 25 to 74% membrane positivity. I think this is going to be a rapidly evolving field the way that we’ve seen HER2-low be defined in breast cancer treated with T-DXd, so we’ll have to stay tuned for more safety and efficacy information related to this drug in breast cancer in particular.
Similar to the safety described in the lung cancer study, in this particular clinical trial, the most frequent AEs were related to cytopenias and there were a couple of patients, 12 I believe, who experienced treatment-related interstitial lung disease according to central adjudication. So, similar to other ADCs, ILD is something that needs to be monitored in patients being treated with this drug.
Dr. Caudle:
Okay, and next let’s talk about zenocutuzumab. Dr. Hurvitz, can you tell me more about the mechanism of action and latest results for this agent?
Dr. Hurvitz:
Zenocutuzumab is a bispecific-targeting HER3 antibody. Zenocutuzumab docks on HER2 and then binds to and blocks the NRG fusion-HER3 interaction and HER3 heterodimerization with HER2. This has been evaluated in breast and lung cancers among 71 patients with measurable disease. Where the investigator-assessed confirmed objective response rate was 34%, including responses observed in non-small cell lung cancer at a rate of 35%, and in a small cohort of breast cancer patients, there were 2 out of 4 patients who had a response observed with a number of patients still ongoing with treatment.
Among 200 in patients treated with this monoclonal antibody monotherapy across all dosing schedules in the phase 2 setting, the grade 3/4 event rate was reported to be less than 5%, which is pretty notable and is perhaps owing to the fact that it’s a monoclonal antibody and not a chemotherapy-loaded ADC.
Dr. Caudle:
Dr. Florez, there are many new agents being developed for HER3, including numerous monoclonal antibodies. Can you walk us briefly through a few of them?
Dr. Florez:
Yes. As I say early, this is the year of HER3 monoclonal antibodies and ADCs. Also, as a thoracic medical oncologist, I’m not used to any of these names, so it feels like we’re competing with hematology now to get the more complicated names of drugs. So, everybody, gear up. Let’s start with lumretuzumab, which is a study in xenograft models of ER-positive, HER2-positive, HER2-low human breast cancers. It was studied in combination with pertuzumab and produced in these xenograft models a potent and long-lasting tumor regression. The efficacy was increased when used in conjunction with fulvestrant. A patient with ER-positive, HER3-positive, HER2-low breast cancer had a prolonged clinical response when it was treated with this triple therapy of lumretuzumab, pertuzumab, and paclitaxel. In addition, two patients were treated with lumretuzumab plus erlotinib for the treatment of lung cancer. The safety of this agent – the main concern that is loss limiting is diarrhea. So, this monoclonal antibody has been associated with significant diarrhea, and, of course, this will be potentiated by chemotherapy. Grade 3 diarrhea produced significant dose reductions in these patients, but we noticed that it was better tolerated after prophylactic loperamide was added to the agent and pertuzumab was omitted. So, there’s still a lot of room for learning how these agents will have a role in the treatment of breast and lung cancer. Another upcoming agent is seribantumab. Seribantumab in combination with trastuzumab inhibits cell growth in HER2-positive breast cancer, including patients that had trastuzumab resistance. Seribantumab also has the antitumoral activity of chemotherapy in HER2-positive breast cancer models that were resistant to paclitaxel and trastuzumab. This agent was overall well tolerated but did not produce any clinical benefit. So, the story may have ended here, but it’s too early to call the benefit or the future of this compound. Finally, we have patritumab. Patritumab alone or in combination with an anti-EGFR monoclonal antibody reduced growth in non-small cell lung cancer xenograft including this very important area, which are patients that have resistance to EGFR TKIs. In addition, the combination of patritumab plus erlotinib helped overcome resistance induced by NRG in non-small cell lung cancer models. For this agent, we didn’t find any dose-limiting toxicities, so – what indicates that the maximum tolerated dose was not reached. The most common adverse events with this included gastrointestinal and skin toxicities, but as for the data that is available, they were overall manageable. So, there are three new agents that we are learning the role and the treatment of breast cancer and lung cancer, particularly after resistance to EGFR TKIs, HER2-directed therapy.
Dr. Caudle:
And what recommendations do you have for healthcare providers who may have patients that would benefit from enrolling in a clinical trial for one of these medications? Dr. Hurvitz, we’ll start with you.
Dr. Hurvitz:
Clinical trial participation is of paramount importance. This is how we move the field forward, so I think it is incredibly important for our healthcare providers in the community, as well as academic centers, to always consider our patients for participation in a clinical trial. I think clinical trial participation is something that can be scary for patients, so I think it warrants a little bit of time in the clinic to explain to patients what clinical trials are about and the regulatory efforts that are involved now to protect clinical trial participants from the abuses that occurred previously. There are so many exciting agents in development right now especially for HER3-expressing or mutated cancers. I think it’s an ideal opportunity as these agents are moving out of phase 1 testing and into phase 2 and 3 testing to consider referring patients to one of these clinical studies.
Dr. Caudle:
Thank you. And Dr. Florez?
Dr. Florez:
Clinical trials should be part of the discussion at day one in the clinic. We need to remove the whole belief that clinical trials are an afterthought after all treatment has been exhausted. A lot of these agents are moving to first line and second line and allows our patients to go on targeted therapy. I often tell my patients I’m not going to run out of docetaxel hopefully, so the opportunity going to clinical trial is now, and I tend to bring up clinical trials at day one so it becomes the norm and patients don’t feel like, oh, I ran out of options so I’m going to be your guinea pig. So, bringing up the conversation very early on.
Dr. Caudle:
And Dr. Hurvitz and Dr. Florez, are there any key take-home messages you would like to share with our audience?
Dr. Hurvitz:
I think it’s a very exciting time as we’re beginning to see therapies that are targeting this antigen that we’ve known has been altered in cancers for some time, so it’s very satisfying to see clinical studies now evaluating ADCs and antibodies that target HER3. It may be that combination therapies with agents that target multiple tumor antigens would improve the outcomes by targeting HER3, and I know a number of studies are underway to do that but I do think that this is a very promising approach, and I’m excited to see more data.
Dr. Florez:
And for me, I think what is exciting is that we consider HER3 and non-small cell lung cancer to be a negative predictive factor, but as we’re developing drugs here, it will stop being bad, and now it will become a targetable agent, and the whole story will be rewritten again. And we have seen this in lung cancer when so many markers were found to be, you know, the bad ones, and now we have targeted therapy for those, and patients are living longer than the ones that they don’t have the biomarker. So, the future is bright, and the numbers – the names of the drugs are complicated, but it’s also extremely academically stimulating to learn all the different ways we can treat cancer now.
Dr. Caudle:
Well, that’s a really great way to round out our discussion, and I’d like to thank our guests, Dr. Sara Hurvitz and Dr. Narjust Florez, for helping us better understand why and how HER3 is targeted in novel treatments for lung and breast cancers. Dr. Hurvitz and Dr. Florez, it was great speaking with you both today.
Dr. Hurvitz:
Thank you so much.
Dr. Florez:
Thank you for the invitation, and hope we get to talk in a few years about what has happened with HER3.
Announcer:
This activity was brought to you by Med-IQ and supported by an educational grant from Daiichi Sankyo, Inc. To receive your free CME credit, be sure to complete the post-test and evaluation at ReachMD.com/CME. This is CME on ReachMD. Be part of the knowledge.














Facebook Comments