Dive in for expert insights for APPs from Dr Roy F. Chemaly, Professor of Medicine in the Department of Infectious Diseases, Infection Control, and Employee Health at The University of Texas MD Anderson Cancer Center in Houston. He’ll discuss the latest clinically relevant data on the prevention and management of cytomegalovirus (CMV) infection in the setting of hematopoietic cell transplant.
Reducing the Burden of Cytomegalovirus in HCT: Optimal Strategy for Prevention
Reducing the Burden of Cytomegalovirus in HCT: Optimal Strategy for Prevention
- Overview of CMV Infection in HCT
- Types and Consequences of CMV Disease After HCT
- Risk Factors for CMV Development After HCT
- Timing and CMV Risk
- PET for CMV
- Managing Side Effects and Toxicity of Antivirals in PET
- Antivirals for CMV Primary Prophylaxis
- Managing Aspects of Prophylaxis With Letermovir
- Decision-making: PET vs Prophylaxis
- Pros and Cons of Prophylaxis Strategy
Overview of CMV Infection in HCT
Return to Table of Contents
Cytomegalovirus (CMV) is a DNA virus and member of the herpesviridae family (also known as β-human herpesvirus type 5). Approximately 50% of the US adult population have been infected with CMV, with a higher prevalence in older patients and among women of childbearing age.1-3 In most individuals, primary CMV infection is largely asymptomatic or mildly symptomatic. After being contained by the immune system, the virus then remains latent in epithelial and other tissues.2,4
Reactivation with CMV is a very common occurrence after allogeneic hematopoietic cell transplant (allo-HCT) due to immunosuppressive conditioning regimens and the high prevalence of latent CMV infection mainly in recipients.5,6 CMV infection reactivates in 60% to 70% of seropositive transplant recipients. Development of primary CMV infection among seronegative transplant recipients who receive a transplant from a seropositive donor has been reported.6
Types and Consequences of CMV Disease After HCT
Return to Table of Contents
Development of CMV disease after CMV reactivation is associated with high morbidity and requires specific treatment to prevent serious complications. Of most concern is development of CMV end-organ disease in the lungs, gastrointestinal tract, eyes, CNS, and other body systems (Table 1). 1,7 In the era before widespread availability of antiviral therapies, CMV pneumonia was the most common end-organ disease after allo-HCT; today, gastrointestinal disease is the most frequent manifestation and pneumonia is second.
Table 1. End-Organ Diseases with CMV1
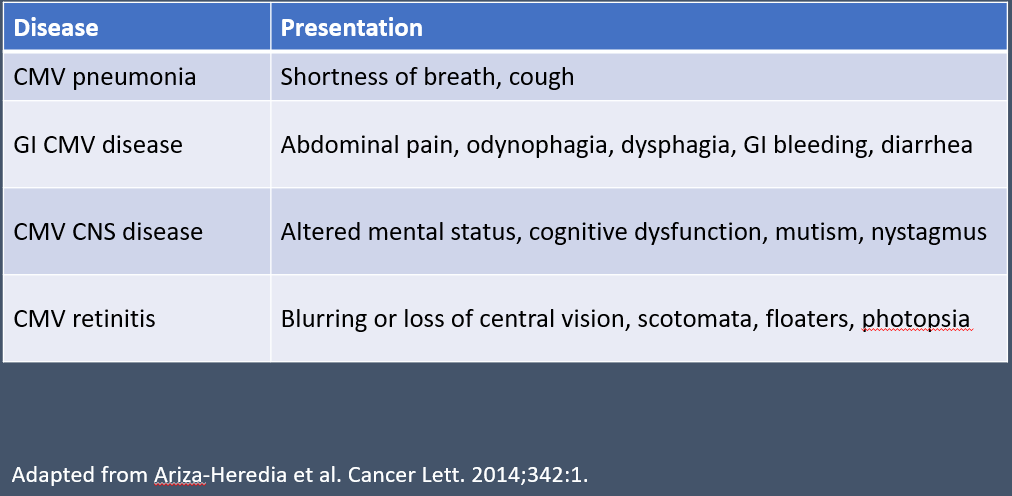
End-organ disease can occur at any time after transplant and remains a concern whenever CMV primary infection or reactivation is present. Disease can be categorized as early onset (within 3 months) or late onset (after 3 months) depending on the time since transplantation.1
In addition to the morbidity associated with CMV disease, ongoing CMV infection itself is associated with additional immunosuppression and secondary infections.1 Of more concern, the development of CMV viremia (ie, detectable viral load) is associated by itself with increased overall and nonrelapse mortality during the year after allo-HCT, even when preemptive therapy (PET) is used.8,9 Use of PET reduced risk for CMV end-organ disease vs no PET, and higher viral loads were associated with greater impact on mortality.8 Mortality is increased independent of the underlying hematologic malignancy being treated with transplantation.9
Therefore, the ideal approach to limit CMV infection following allo-HCT is to control it early or prevent it entirely. The only effective nonpharmacologic strategy for prevention of CMV infection is to match a seronegative donor and seronegative recipient.7 Perhaps counterintuitively, matching a seronegative donor with a seropositive recipient is associated with negative outcomes, including poorer survival.7 With few nonpharmacologic preventive options, 2 pharmacologic strategies are widely used: PET and prophylaxis.10
Risk Factors for CMV Development After HCT
Return to Table of Contents
The risk for CMV reactivation and disease generally is higher among patients who are CMV seropositive prior to allo-HCT, as the immunosuppressive aspects of the HCT process trigger latent CMV to reactivate. Recipient seropositivity is the most important predictor of post-transplantation CMV risk, and without prophylaxis, up to 80% of seropositive patients may develop CMV reactivation after transplantation.1,7
All patients should be tested for latent CMV by serology using immunoglobulin G (IgG) prior to transplant.7 CMV status is classified as a primary infection, active infection, seropositive, or reactivated as below:
- Primary infection: new CMV infection in an individual previously seronegative for CMV
- Seropositive: Latent infection defined as presence of CMV-specific IgG in the absence of active viral replication7,11
- Reactivation: Development of detectable virus in blood after a period of latent seropositivity; reactivation is not the equivalent of primary CMV infection7
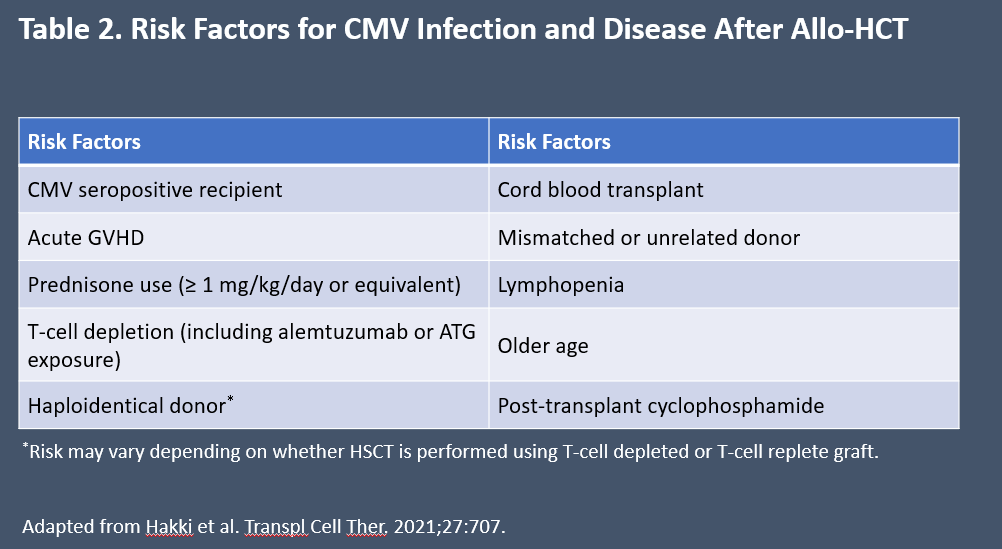
Risk factors for CMV infection or reactivation after transplant include low lymphocytes count. Presence of CD4+ T-cells <50/mL at 3 months after transplant is a risk factor for development of late-onset CMV disease.6 Other factors that increase risk are listed in Table 2.7
Table 2. Risk Factors for CMV Infection and Disease After Allo-HCT7
Timing and CMV Risk
Return to Table of Contents
The factors that affect risk for CMV are dynamic, in the sense that different factors are associated with increased risk at different time points after transplantation. Risk for CMV can be thought of in 3 phases: Days 0 to 30 post-HCT, Days 30 to 99, and Day 100+. In the 30 days right after transplantation, key risk factors are the type of conditioning chemotherapy used prior to transplant (myeloablative regimens having highest risk), as well as older age and recipient seropositivity (Figur e 1).1 Use of post-transplant cyclophosphamide or high-dose anti-thymocyte for T-cell depletion, which effectively reduces risk for acute GVHD also increases risk for CMV infection.2 Use of matched unrelated donors or haploidentical donors is associated with higher risk for CMV reactivation compared with matched related donors. Cord-blood transplant also increases risk, as the naive T-cells in cord blood take longer to restore antigen-specific immunity.2
Figure 1. CMV risk factors and presentation over time from allo-HCT.1
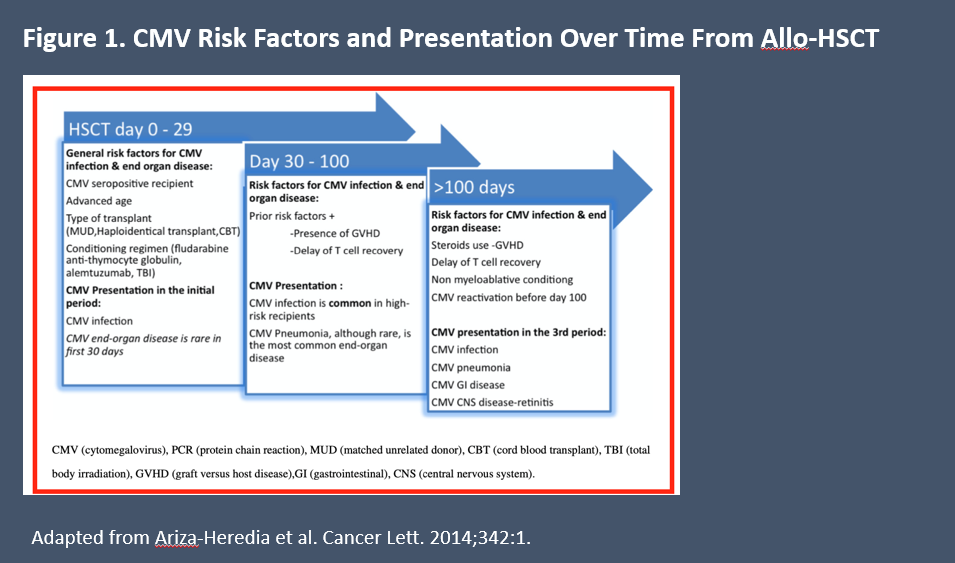
In the intermediate period, risk factors include GVHD development and steroid use as treatment. Risk factors in the late time period include chronic steroid use, chronic GVHD, and delayed T-cell recovery. It is also during the intermediate and late phases that CMV end-organ disease tends to manifest.1
Donor positivity can affect risk for CMV infection but it is not currently a factor in treatment decisions in guidelines. One European study suggests increased mortality with transplant from an unrelated donor whose CMV status is opposite the recipient’s, but this is controversial. In contrast, transplants between CMV-seropositive recipients and seropositive unmatched donors improved overall survival (OS) vs seronegative donors in that study.12
PET for CMV
Return to Table of Contents
PET is based on the concept of weekly (or twice weekly) monitoring for CMV reactivation for up to 100 days after transplant, using quantitative polymerase chain reaction (qPCR) to check CMV viral load. The aim is to prevent progression to CMV end-organ disease. The CMV viral load that triggers therapy varies among transplant centers, but typically ranges from about 150 to 1500 IU/mL depending on the patient’s risk status.7,13 In essence, PET is predicated upon monitoring CMV reactivation and then intervening early to prevent development of CMV disease.7
Of the 2 common methods for monitoring CMV viral levels, the ASTCT prefers qPCR over pp65 antigen testing because of PCR’s greater sensitivity. When required, all PET starts with induction dosing and then transitions to secondary prophylaxis (the term preferred by ASTCT) with viral clearance or significant reduction in viral load.7 Dosages used in secondary prophylaxis generally are lower than induction, which may mitigate treatment toxicity.
Ganciclovir or valganciclovir are the preferred first-line choices for PET and are usually effective.2,13 Of these agents, only ganciclovir is FDA approved for CMV-related uses in patients after allo-HCT; valganciclovir is approved after solid organ transplant.7,14,15 Treatment duration is usually between 2 to 3 weeks or until CMV viral load is undetectable.13
Managing Side Effects and Toxicity of Antivirals in PET
Return to Table of Contents
Myelosuppression is the major toxicity of ganciclovir and valganciclovir, which impacts efficacy and increases risk for secondary infections. Neutropenia with ganciclovir usually develops during the first 2 weeks of treatment but can develop at any time with ganciclovir or valganciclovir. Neither drug is recommended for patients with existing absolute neutrophil counts (ANC) <500 cells/µL, hemoglobin <8 g/dL, or platelets <25,000 cells/µL.14,15 In clinical trials with allo-HCT patients who received ganciclovir, ANC <500 cells/µL occurred in 12% and a total of 41% had ANC <1000 cell/µL; a total of 51% had platelets <50,000 cells µL, including 31% with platelets <25,000 cells/µL.14 In clinical trials with valganciclovir for CMV retinitis, ANC <500 cells/µL occurred in 19% of patients.15
In a comparison of valganciclovir with ganciclovir for PET following HCT, no severe side effects were reported. Patients who received ganciclovir were more likely to require erythrocyte transfusion, but the difference was not significant.16 The most frequent nonhematologic side effects with valganciclovir include diarrhea, fever, fatigue, headache, insomnia, urinary tract infection/upper respiratory tract infection, and vomiting.15 Valganciclovir has the advantage of oral administration, whereas ganciclovir, foscarnet, and the others are IV drugs.7
Foscarnet has also been used for PET and does not cause myelosuppression. The most concerning toxicity with foscarnet is nephrotoxicity and electrolytes imbalance, with 14% of patients experiencing severe renal impairment in clinical trials.17 The drug directly damages renal tubular cells. Toxicity usually develops within 2 weeks of induction, but can occur at any time, and patients must be monitored regularly while on treatment. Foscarnet should be avoided for patients with existing renal impairment.17,18 Another toxicity of special concern is electrolytes imbalances—principally in potassium and magnesium—that lowers seizure thresholds and requires regular monitoring. Hydration with normal saline or dextrose solution prior to or during infusion may reduce these risks; patients require close monitoring and rapid treatment to correct electrolytes imbalances should they occur.17,18 Other side effects that have been reported with foscarnet include QT prolongation, hypersensitivity reactions, and dizziness. Patients should have ECG and electrolyte measurement prior to starting treatment and at intervals on treatment.17
Valganciclovir and ganciclovir have boxed warnings for hematologic toxicity (including bone marrow failure), fertility impairment in males and females, and fetal toxicity.14,15 Foscarnet has boxed warnings for renal impairment and potential for seizures related to electrolyte imbalances.17
Maribavir is an oral drug that has demonstrated efficacy similar to valganciclovir in PET. It is associated with dysgeusia and some gastrointestinal toxicity. The most common gastrointestinal toxicity was dysgeusia, reported in from 15% to 82% of clinical trial participants. It has a very low rate of neutropenia and no significant nephrotoxicity or marrow toxicity.18,19 Maribavir is FDA approved for treatment of refractory CMV infection with or without genotypic resistance post-transplant.19
In contrast, cidofovir is a once-weekly IV drug associated with risk of nephrotoxicity and renal tubular damage that limit its utility for PET. It is more often reserved for treatment of resistant CMV infections.18
Antivirals for CMV Primary Prophylaxis
Return to Table of Contents
Historically, antivirals used to suppress CMV were associated with significant toxicity, including severe nephropathy and myelosuppression that increased infection risk. These toxicities made PET a more acceptable option than universal prophylaxis. For example, acyclovir and valacyclovir have poor efficacy. Ganciclovir, valganciclovir, and foscarnet have significant toxicity; and phase III studies of maribavir and brincidofovir for prophylaxis did not meet the primary endpoints.20 However, with better understanding of the risks of CMV viremia and availability of letermovir, which is highly effective and well-tolerated, practice has shifted toward prophylaxis.2,6,9,10,13,20 Letermovir uniquely targets the viral terminase complex, and avoids toxicities associated with other antivirals and limits risk for cross-resistance.21
Letermovir efficacy was established in a phase III study that compared it with placebo for prophylaxis in 565 CMV-seropositive patients undergoing HCT.22 In the study, prophylaxis was initiated a median 9 days after transplantation and continued through Week 14. At Week 24, 37.5% of those receiving letermovir and 60.6% of those receiving placebo had developed csCMVi requiring preemptive treatment. Significant reduction in CMV reactivation was seen in both high-risk and low-risk subgroups.22
Prophylaxis with letermovir improved OS vs placebo following HCT in a prespecified exploratory analysis at 24 weeks of follow-up: 10.2% (95% CI: 6.8-13.6) in the letermovir arm vs 15.9% (95% CI: 10.2-21.6) in the placebo arm (P = .03) (Figure 2).22 At 48 weeks, all-cause mortality in patients who received letermovir prophylaxis was lower than among those who received placebo (20.9% vs 25.5%, respectively, P = .12).22 In another analysis to assess all-cause mortality through Week 48 following letermovir prophylaxis, the hazard ratio was 0.74 (95% CI: 0.49-1.11, P = .14) vs placebo.23 In addition, prophylaxis with letermovir significantly reduced the risk for development of csCMVi after HCT, suggesting that its OS benefit could be related to reduction in CMV reactivation.23 In a meta-analysis of real-world data, letermovir was associated with 27% reduction in risk for all-cause mortality and 35% reduction in risk for nonrelapse mortality beyond 200 days after transplantation.24
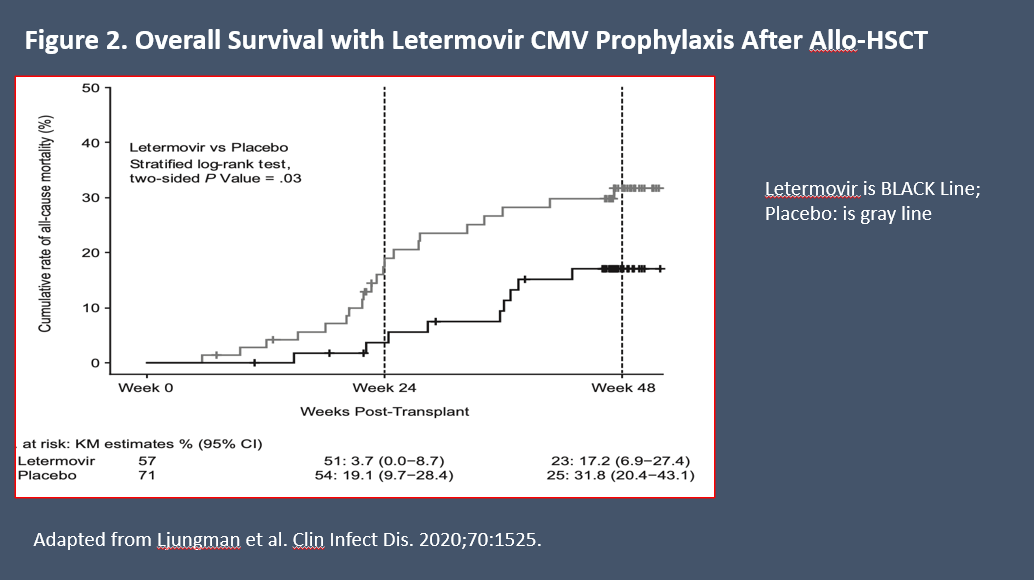
A single-center retrospective comparative study of 537 consecutive CMV-seropositive HCT recipients found that primary prophylaxis with letermovir was associated with reductions in CMV end-organ disease in the lungs and gastrointestinal tract; reductions in antiviral-resistant or -refractory CMV infections; and lower nonrelapse mortality at Week 48 compared with those not receiving letermovir.25 Subsequently, a meta-analysis of real-world data with letermovir for primary prophylaxis, which included observational studies with 7104 patients who underwent allo-HCT was done. In the analysis, primary prophylaxis with letermovir vs no prophylaxis (with or without PET) was associated with significant reductions in CMV reactivation and reductions in development of csCMVi and CMV disease by Day 100 and Day 200 after transplantation.24
In the phase III trial, approximately 10% of all patients and 20% of high-risk patients developed csCMVi after discontinuation of letermovir prophylaxis around Day 100, suggesting longer-duration prophylaxis could be beneficial.7 The optimal duration of letermovir prophylaxis was studied in a phase III trial comparing rates of csCMVi during post-transplant Weeks 14 to 28 (~100-200 days). HCT patients considered at high risk for CMV were randomly assigned to continue letermovir prophylaxis after Day 100 or switch to placebo. Patients who received 200 days of letermovir were less likely to develop csCMVi by Week 28 (2.8% vs 18.9%), and less likely to develop CMV end-organ disease. Treatment failures (csCMVi + discontinuations) occurred in 2.8% with extended letermovir vs 18.9% with placebo (P = .0005).26
Although highly effective in reducing csCMVi and disease, widespread use of letermovir for primary prophylaxis may raise concerns about antiviral resistance. To address these concerns, some centers have explored a risk-based strategy for primary prophylaxis. Using this approach, 1 single-center study concluded that risk-based letermovir primary prophylaxis effectively protected those at high risk, while allowing most low-risk patients to forego prophylaxis with minimal rates of CMV reactivation, relapse, and development of serious CMV infection.27
At present, letermovir is approved only for use in adult patients, although it is used in some pediatric centers. A retrospective review of 14 pediatric patients (median age 12 years) who received letermovir prophylaxis concluded that it appears to be well-tolerated and effective.28 A clinical trial in pediatric patients undergoing allo-HCT is ongoing (NCT03948506), and preliminary results in the adolescent age subgroups indicate it is safe and effective.29 Letermovir is not recommended for use in PET strategies given its lower barrier of resistance.7
Only letermovir and ganciclovir are approved specifically for prophylaxis in CMV-seropositive patients undergoing transplantation.14,30 When used as primary prophylaxis, ganciclovir is highly effective against many herpesviruses, but has no survival benefit and the same toxicity issues seen when used in PET, including myelosuppression and secondary bacterial and fungal infections.4 In prophylaxis, it appears that ganciclovir did not improve survival due to the effects of severe neutropenia and secondary fungal infections.6 It was studied in randomized trials as prophylaxis vs placebo in seropositive allo-HCT recipients and significantly reduced rates of CMV infection and CMV disease. In 1 of these studies, ganciclovir-related neutropenia led to treatment interruption in 58% of patients.31 In another study with seronegative recipients, ganciclovir reduced CMV infection and disease, but treatment-associated neutropenia led to higher rates of bacterial infection.32
Neither valganciclovir nor maribavir improved outcomes as prophylaxis. Maribavir was not superior to placebo in a phase III trial, and valganciclovir as prophylaxis did not improve outcomes vs its use in a PET strategy (while still associated with myelosuppression).4,7,13,19,33 The strategy of using prophylactic IV immunoglobulin or CMV-enriched IgG has not demonstrated benefit and its use is not recommended.7
Managing Aspects of Prophylaxis With Letermovir
Return to Table of Contents
The most concerning aspect of treatment with letermovir is risk for clinically relevant drug–drug interactions. Letermovir is a substrate and inhibitor of several key pathways, including OATP 1B1/3, UGT1A1/3, and efflux transporters (eg, P-glycoprotein).21,30 Coadministration with drugs that are CYP3A substrates or are OATP1B1/3 substrates may increase concentration of those drugs. This affects a substantial number of other drugs in various ways; in transplantation, letermovir can reduce or increase the effects of immunosuppressant drugs and extended-spectrum azole drugs. Conversely, coadministration of letermovir with some drugs may reduce letermovir effect. The complete list of drug–drug interactions is extensive and should be reviewed before treatment planning.21,30
In a phase III trial, more patients receiving letermovir (71.0%) than placebo (41.2%) completed the trial regimen through Week 14. Similar percentages of patients experienced adverse events (any: 97.9% vs 100%, respectively), including nausea, which was the most frequent reason for discontinuation (26.5% vs 23.4%), diarrhea (26.0% vs 24.5%), vomiting (18.5% vs 13.5%), headache (13.9% vs 9.4%), and others.22 Another frequent side effect is peripheral edema, which was reported in 14% of patients receiving letermovir and 9.4% receiving placebo.30 Hematologic and renal toxicity occurred in similar percentages of patients treated with letermovir and placebo.22,30 Overall, 19% of patients in each group developed severe neutropenia (<500 cells/µL). Other outcomes with letermovir vs placebo, respectively, were hemoglobin <6.5 g/dL 2% vs 1%; platelets <25,000 cells/µL 27% vs 21%; and serum creatinine >2.5 mg/dL 2% vs 3%. Severity of adverse events was similar with letermovir or placebo, and similar percentages discontinued due to side effects (13% vs 12%).30 In long-term follow-up of patients who received letermovir, no additional toxicities have emerged.
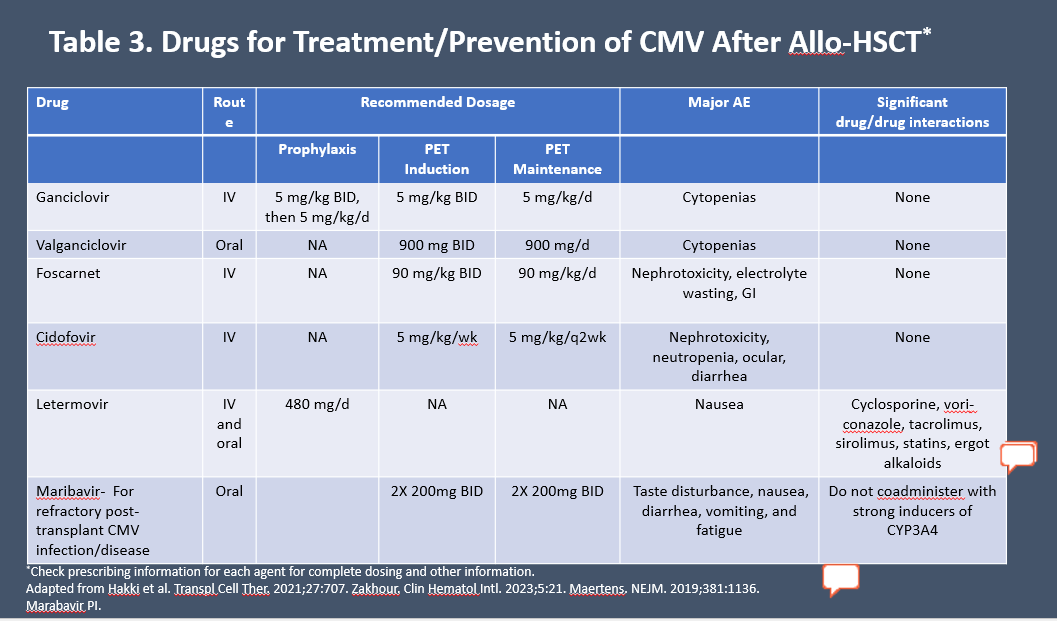
Currently recommended agents for treatment and prevention are summarized in Table 3.7,13,34,35
Table 3. Drugs for Treatment/Prevention of CMV After Allo-HCT*7,13,34,35
Decision-making: PET vs Prophylaxis
Return to Table of Contents
According to guidelines from ASTCT, PET and prophylaxis are complementary approaches to CMV disease prevention, and all centers should be equipped for both.7 Among the benefits of a PET strategy are the potential to reduce unnecessary treatment with antivirals and associated toxicity and resistance development.7,20 Resistance development is a concern because several antivirals share major resistance mutations (eg, UL54), including first-line choices ganciclovir and valganciclovir, as well as foscarnet and cidofovir. Newer agents, including maribavir and letermovir, are associated with different mutations, limiting cross-resistance.21
PET strategies also have a long history with a large body of evidence, providing confidence in their use. However, most factors favor a prophylaxis strategy with letermovir if available. Among the caveats with PET strategies is the demonstrated effect of even minor CMV reactivation in the 100 days following HCT on development of end-organ disease, risk for acute or chronic GVHD and invasive fungal infections, and poorer transplant outcomes including mortality.6,8,9
There is also the chance that rapid viral load doubling time may outpace the antiviral effect of PET intervention in some patients.10 In addition, while a PET strategy may reduce risk for early-onset CMV disease, patients remain at risk for late-onset and development of refractory or resistant CMV infection.6 Finally, logistic and cost difficulties can hamper effective PET strategies, including the need for ongoing monitoring for 100+ days.10,20
Pros and Cons of Prophylaxis Strategy
Return to Table of Contents
There are many positives associated with using letermovir for primary prophylaxis of patients undergoing allo-HCT. As noted previously, this strategy improves survival in CMV-seropositive transplant recipients, and reduces risk for CMV disease and development of resistant or refractory infection.10,22,23,25 However, 1 caveat with letermovir use is that it is effective only against CMV, and not effective against herpes simplex virus (HSV) or varicella zoster virus (VZV); additional prophylaxis with acyclovir may be needed.7,21
Myelotoxicity with ganciclovir or valganciclovir makes primary prophylaxis a less effective choice, as this toxicity may limit efficacy due to dose adjustments or treatment interruptions.20
Prophylactic vs PET strategies also may delay reconstitution of CMV-specific immunity, which tends to recover more quickly with PET.10,20 Further, any prophylactic strategy includes the risk for unnecessary use of antivirals, which is balanced by improved survival. As the strategy evolves with newer options, a risk-based approach may mitigate the concern about excess antiviral use.27
Current guidelines from ASTCT and the National Comprehensive Cancer Network recommend prophylaxis with letermovir, particularly in seropositive recipients. Prophylaxis should begin within 28 days of transplantation and continue through Day 100 or longer, depending on patient factors.7,36
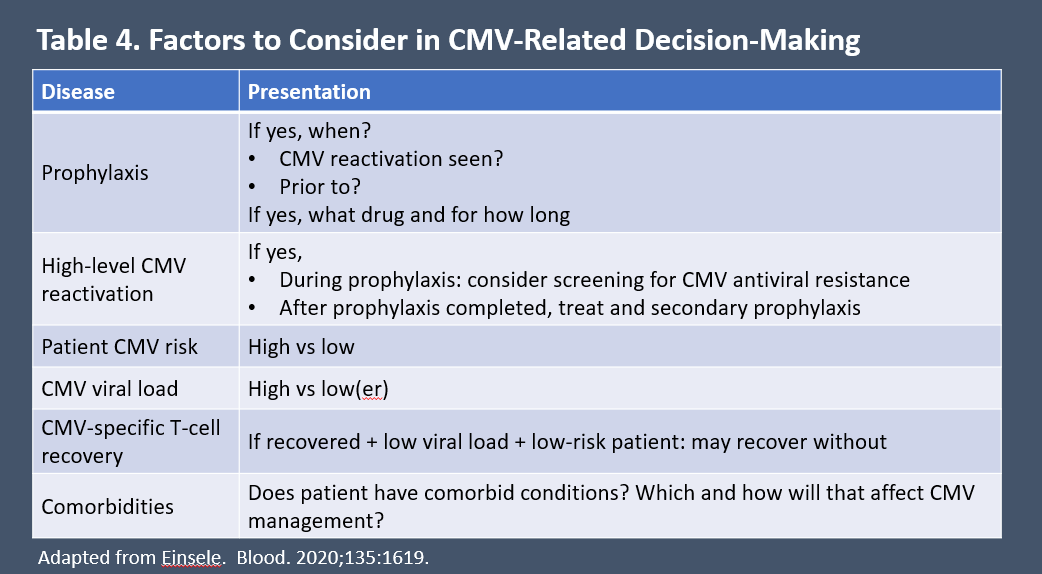
Factors that can help guide CMV-related decision-making are listed in Table 4.6 Patients who receive letermovir (or other) prophylaxis and develop CMV infection usually are managed with a PET strategy.
Table 4. Factors to Consider in CMV-Related Decision-making6
Whichever strategy is chosen, patient engagement is key to its success. Engaged patients who are informed and involved in decision-making are more likely to accept the rigors of treatment. Patients and their caregivers should be educated about the impact of CMV infection, the rationale for PET or prophylaxis, its requirements, and expected toxicities.
Patient preference for oral vs IV therapy is a consideration. Letermovir is available orally and intravenously; ganciclovir is administered intravenously once or twice daily, requiring venous access. Valganciclovir is an oral drug with twice-daily dosing. For PET, patients must be available for qPCR monitoring weekly for at least 3 months after their procedure, and willing to accept therapy if CMV reactivation is detected. Those treated with oral agents need understanding of the importance of adherence to treatment efficacy, and strategies to support adherence. Education on expected toxicities and differentiating those requiring intervention can help to avoid treatment discontinuation due to mild or moderate nausea or gastrointestinal distress. However, treatment absorption may be decreased if severe vomiting or diarrhea occur. Patients should know to contact their healthcare team in the event of severe vomiting or diarrhea.
A successful outcome is more likely if education about CMV disease prevention is presented as an integral part of the HCT procedure and included prior to the transplant itself.
On the Horizon
Vaccine research is an area of intense interest in CMV prevention. Following a prior history of unsuccessful vaccine trials in HCT patients, several promising candidates have reported results. One is an mRNA-based CMV vaccine (mRNA-1647), studied to date in a randomized, placebo-controlled phase II study in healthy adults. Seronegative or seropositive individuals were included, with efficacy endpoints including development of antigen-specific antibodies and neutralizing titers. In the study, mRNA-11647 was immunogenic in CMV-seronegative individuals, causing a robust antibody response, and boosting neutralizing antibody titers in seropositive individuals. The vaccine was well-tolerated and is moving to phase III development.37 Another vaccine candidate using a modified vaccinia Ankara vector (Triplex) to develop CMV-specific T-cell response was studied in a phase II trial that enrolled 102 CMV-seronegative HCT recipients. Vaccination reduced the rate of CMV reactivation vs placebo, 9.8% vs 19.6%, respectively. The vaccine was safe, with no grade ≥3 toxicities.5,10 A phase I trial of Triplex vaccinated healthy matched related donors prior to HCT, of whom 16 of 17 were CMV seropositive prior to vaccination. The strategy led to early and robust CMV-specific T-cell reconstitution in recipients, all of whom were CMV seropositive. Vaccination was well tolerated. The study is now ongoing in phase II (NCT03560752).38
A complex procedure called adoptive T-cell therapy or adoptive immunotherapy involves generating CMV-specific T-cells, then expanding them either in vitro or by infusing them into the transplant recipient. The process has been evaluated in several small studies, which suggest it is an effective approach to prophylaxis.2 However, it is still investigational and likely will be reserved for resistant or refractory CMV infection if it becomes available.2,7
References
- Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett. 2014;342:1-8.
- Cui J, Zhao K, Sun Y, et al. Diagnosis and treatment for the early stage of cytomegalovirus infection during hematopoietic stem cell transplantation. Front Immunol. 2022;13:971156.
- Fowler K, Mucha J, Neumann M, et al. A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development. BMC Public Health. 2022;22:1659.
- Chen K, Cheng MP, Hammond SP, Einsele H, Marty FM. Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Adv. 2018;2:2159-2175.
- Aldoss I, La Rosa C, Baden LR, et al. Poxvirus vectored cytomegalovirus vaccine to prevent cytomegalovirus viremia in transplant recipients: a phase 2, randomized clinical trial. Ann Intern Med. 2020;172:306-316.
- Einsele H, Ljungman P, Boeckh M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. 2020;135:1619-1629.
- Hakki M, Aitken SL, Danziger-Isakov L, et al. American Society for Transplantation and Cellular Therapy series: #3-prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27:707-719.
- Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3:e119-127.
- Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127:2427-2438.
- Haidar G, Boeckh M, Singh N. Cytomegalovirus infection in solid organ and hematopoietic cell transplantation: state of the evidence. J Infect Dis. 2020;221:S23-S31.
- Bulka CM, Bommarito PA, Aiello AE, Fry RC. Cytomegalovirus seroprevalence, recurrence, and antibody levels: Associations with cadmium and lead exposures in the general United States population. Environ Epidemiol. 2020;4:e100.
- Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59:473-481.
- Zakhour J, Allaw F, Haddad SF, Kanj SS. The ten most common questions on cytomegalovirus infection in hematopoietic stem cell transplant patients. Clin Hematol Int. 2023;5:21-28.
- Ganciclovir [prescribing information]. Schaumberg, IL: Sagent Pharmaceuticals; 2020.
- Valganciclovir [prescribing information]. South San Francisco, CA: Genentech USA, Inc.; 2021.
- van der Heiden PL, Kalpoe JS, Barge RM, Willemze R, Kroes AC, Schippers EF. Oral valganciclovir as pre-emptive therapy has similar efficacy on cytomegalovirus DNA load reduction as intravenous ganciclovir in allogeneic stem cell transplantation recipients. Bone Marrow Transplant. 2006;37:693-698.
- Foscarnet [prescribing information]. Lake Forest, IL: Hospira, Inc; 2020.
- Jakharia N, Howard D, Riedel DJ. CMV infection in hematopoietic stem cell transplantation: prevention and treatment strategies. Curr Treat Options Infect Dis. 2021;13:123-140.
- Halpern-Cohen V, Blumberg EA. New perspectives on antimicrobial agents: maribavir. Antimicrob Agents Chemother. 2022;66:e0240521.
- Stycynski J. Prophylaxis vs preemptive therapy for prevention of CMV infection: new insight on prophylactic strategy after allogeneic hematopoietic cell transplantation. Acta Haematol Polonica. 2020;51:17.
- Saullo JL, Miller RA. Cytomegalovirus therapy: role of letermovir in prophylaxis and treatment in transplant recipients. Annu Rev Med. 2023;74:89-105.
- Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433-2444.
- Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis. 2020;70:1525-1533.
- Vyas A, Raval AD, Kamat S, LaPlante K, Tang Y, Chemaly RF. Real-world outcomes associated with letermovir use for cytomegalovirus primary prophylaxis in allogeneic hematopoietic cell transplant recipients: a systematic review and meta-analysis of observational studies. Open Forum Infect Dis. 2023;10:ofac687.
- Sassine J, Khawaja F, Shigle TL, et al. Refractory and resistant cytomegalovirus after hematopoietic cell transplant in the letermovir primary prophylaxis era. Clin Infect Dis. 2021;73:1346-1354.
- Dadwal SS, Russo D, Stelljes M, et al. A phase 3 randomized, double-blind, placebo-controlled trial evaluating the safety and efficacy of letermovir prophylaxis when extended from 100 to 200 days post-transplant in cytomegalovirus-seropositive recipients of an allogeneic hematopoietic stem cell transplant. Presented at: ASTCT/CIBMTR World Congress, February 18, 2023. tandem.confex.com/tandem/2023/meetingapp.cgi/Paper/21616. Accessed July 13, 2023.
- Sourisseau M, Faure E, Behal H, et al. The promising efficacy of a risk-based letermovir use strategy in CMV-positive allogeneic hematopoietic cell recipients. Blood Adv. 2023;7:856-865.
- Korholz KF, Fuller MA, Hennies M, et al. Letermovir for prophylaxis and pre-emptive therapy of cytomegalovirus infection in paediatric allogeneic haematopoietic cell transplant patients. Paediatr Drugs. 2023;25:225-232.
- Groll AH, Schulte JH, Antmen AB, et al. Preliminary dosing for adolescent hematopoietic stem-cell transplant (HSCT) recipients based on pharmacokinetic (PK), safety, and efficacy data of letermovir (LET) for cytomegalovirus (CMV) prophylaxis. Open Forum Infect Dis. 2022;Supplement_2:ofac492.675.
- Letermovir [prescribing information]. Rahway, NJ: Merck & Co, Inc.; 2022.
- Winston DJ, Ho WG, Bartoni K, et al. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993;118:179-184.
- Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993;118:173-178.
- Boeckh M, Nichols WG, Chemaly RF, et al. Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial. Ann Intern Med. 2015;162:1-10.
- Maertens J, Cordonnier C, Jaksch P, et al. Maribavir for preemptive treatment of cytomegalovirus reactivation. N Engl J Med. 2019;381:1136-1147.
- Maribavir [prescribing information]. Lexington, MA: Takeda Pharmaceuticals America, Inc.; 2023.
- National Comprehensive Cancer Network. Prevention and treatment of cancer-related infections. Version 3.2022. www.nccn.org.
Disclosure of Conflicts of Interest
Partners for Advancing Clinical Education (PACE) requires instructors, planners, managers, and other individuals who are in a position to control the content of this activity to disclose all financial conflicts of interest (COI) they may have with ineligible companies. All relevant COI are thoroughly vetted and mitigated according to PACE policy. PACE is committed to providing its learners with high-quality CME/CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
The faculty reported the following relevant financial relationships or relationships to products or devices they have with ineligible companies related to the content of this educational activity:
Primary Author
Roy F. Chemaly, MD, MPH
Professor of Medicine
Department of Infectious Diseases, Infection Control, and Employee Health
The University of Texas MD Anderson Cancer Center
Houston, TexasRoy F. Chemaly, MD, MPH:consultant/advisor/speaker: ADMA Biologics, AiCuris, Karius, Qiagen, Takeda; researcher: AiCuris, Ansun Pharmaceuticals, Karius, Merck, Takeda, Viracor; independent contractor: Merck.
The planners and content peer reviewers from Partners for Advancing Clinical Education and Practicing Clinicians Exchange do not have any relevant financial relationships to disclose.
Target Audience
This activity is intended for NPs and PAs who manage HSCT patients who are at risk for CMV infection.
Learning Objectives
Upon completion of this activity, participants should be able to:
- Describe risk factors for CMV infection among HCT recipients
- Apply guideline recommendations for use of prophylactic or preemptive strategies to prevent CMV
- Evaluate evidence supporting use of therapies for CMV in HCT
Accreditation and Credit Designation Statements
Joint Accreditation Statement
 In support of improving patient care, Partners for Advancing Clinical Education (PACE) is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the healthcare team.
In support of improving patient care, Partners for Advancing Clinical Education (PACE) is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the healthcare team.ANCC Credit Designation
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.0 contact hour, including 1.0 hour of pharmacology credit.AAPA Credit Designation
 Partners for Advancing Clinical Education (PACE) has been authorized by the American Academy of PAs (AAPA) to award AAPA Category 1 CME credit for activities planned in accordance with AAPA CME Criteria. This activity is designated for 1 AAPA Category 1 CME credit. Approval is valid until July 20, 2024. PAs should only claim credit commensurate with the extent of their participation.
Partners for Advancing Clinical Education (PACE) has been authorized by the American Academy of PAs (AAPA) to award AAPA Category 1 CME credit for activities planned in accordance with AAPA CME Criteria. This activity is designated for 1 AAPA Category 1 CME credit. Approval is valid until July 20, 2024. PAs should only claim credit commensurate with the extent of their participation.
IPCE Credit Designation This activity was planned by and for the healthcare team, and learners will receive 1 Interprofessional Continuing Education (IPCE) credit for learning and change.
This activity was planned by and for the healthcare team, and learners will receive 1 Interprofessional Continuing Education (IPCE) credit for learning and change. Disclosure ogf Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Provider(s)/Educational Partner(s)

Provided by Partners for Advancing Clinical Education (PACE)Commercial Support
Supported by an educational grant from Merck Sharp & Dohme, LLC.
Publication Dates
Release Date:
Expiration Date:
Recommended
First-Line Treatment of Metastatic Melanoma After Adjuvant Anti-PD-1
First-Line Treatment of Metastatic Melanoma After Adjuvant Anti-PD-1
MinuteCE®First-Line Treatment of Metastatic Melanoma After Adjuvant Anti-PD-1
0.75 program credits0.75 program credits- 0.75 credits 7 episodes
Optimizing Therapeutic Selection for a Patient With Newly Diagnosed BRAF V600e-Mutated Metastatic Melanoma
Optimizing Therapeutic Selection for a Patient With Newly Diagnosed BRAF V600e-Mutated Metastatic Melanoma
MinuteCE®Optimizing Therapeutic Selection for a Patient With Newly Diagnosed BRAF V600e-Mutated Metastatic Melanoma
0.75 program credits0.75 program creditsPatient Case: Tailoring First-Line Treatment for a Patient With Metastatic Melanoma and Impaired Performance Status
Patient Case: Tailoring First-Line Treatment for a Patient With Metastatic Melanoma and Impaired Performance Status
MinuteCE®Patient Case: Tailoring First-Line Treatment for a Patient With Metastatic Melanoma and Impaired Performance Status
0.75 program credits0.75 program credits











Facebook Comments